When you pick up a prescription for insulin or a biologic drug like Humira, you might not realize the pill you’re handed could be a completely different product than what your doctor wrote on the script-and that’s by design. In the United States, some biosimilars aren’t just similar to the original biologic-they’re officially labeled interchangeable, meaning pharmacists can swap them out without asking your doctor first. This isn’t like generic pills you’ve taken for years. Biosimilars are far more complex, made from living cells, not chemicals. And the rules around who can switch them, when, and how are anything but simple.
What Makes a Biosimilar Interchangeable?
Not all biosimilars are created equal. The FDA approves two types: biosimilars and interchangeable biosimilars. Both must prove they work the same way as the original biologic drug. But only interchangeable ones have passed extra tests. These tests show that switching back and forth between the original drug and the biosimilar-multiple times-doesn’t increase the risk of side effects or reduce how well the treatment works. That’s the key difference. For example, Semglee, an interchangeable insulin, was tested in patients who switched between it and Lantus (the original) several times. No safety issues showed up. That’s what earned it the "interchangeable" label. The FDA doesn’t call interchangeable biosimilars "better." They’re just approved for automatic substitution. All FDA-approved biosimilars, whether interchangeable or not, meet the same high bar for safety and effectiveness. The extra studies for interchangeability are about the switching process, not the drug’s quality.How Is This Different From Generic Drugs?
Think of generic pills like aspirin or metformin. They’re exact chemical copies of brand-name drugs. Because they’re simple molecules, the FDA can prove they’re identical in the body with just a few blood tests. That’s why pharmacists can swap them freely under the Hatch-Waxman Act. Biosimilars are different. They’re made from living cells-like bacteria or yeast-which means no two batches are exactly alike. Even tiny changes in how they’re made can affect how they behave in the body. That’s why the FDA requires more proof. A biosimilar isn’t a copy. It’s a very close match. And for automatic substitution, it must prove it can be switched multiple times without causing problems.Which Biosimilars Are Interchangeable?
As of late 2023, only 10 out of 41 approved biosimilars in the U.S. have interchangeable status. The first was Semglee (insulin glargine-yfgn), approved in July 2021. It’s now used by thousands of people with diabetes who save hundreds per month compared to Lantus. The first interchangeable monoclonal antibody was Cyltezo (adalimumab-adbm), approved in August 2023, a biosimilar to Humira. Other interchangeable biosimilars include Hyrimoz and Hadlima for adalimumab, and Ogivri for trastuzumab. But here’s the catch: interchangeability only applies to swapping with the original reference product. If you’re on one biosimilar and your pharmacist wants to switch you to another biosimilar of the same drug, that’s not allowed without your doctor’s approval. The FDA hasn’t approved "biosimilar-to-biosimilar" switching. This creates confusion-especially when multiple interchangeable options exist.
State Laws Make It Complicated
Even if the FDA says a biosimilar is interchangeable, your pharmacist might still need your doctor’s permission to switch you. That’s because each state has its own rules. Forty states let pharmacists substitute interchangeable biosimilars without telling the doctor first. Arizona, for example, lets pharmacists swap automatically but requires them to notify you and your doctor in writing within five days. But in four states-Alabama, Indiana, South Carolina, and Washington-and in Washington D.C., pharmacists can’t substitute at all unless the doctor says it’s okay. Six states, including Arkansas and Ohio, only allow substitution if it saves you money. And in some states, insurance companies force substitution automatically, even if you’d prefer to stick with the original. This patchwork of laws makes life hard for national pharmacy chains. A pharmacist in California might have to check if a substitution saves money. One in Arizona doesn’t. Their computer systems don’t always know the difference. A 2022 survey found that 67% of independent pharmacists are confused about what they’re allowed to do.What Do Patients Really Experience?
Some patients report no issues. One person with psoriasis switched from Humira to Hyrimoz and saved $800 a month with no change in symptoms. Another switched to Hadlima without being told-and had an allergic reaction. Turns out, the excipient (a non-active ingredient) in the biosimilar was different from what they’d taken before. A 2022 survey by the National Psoriasis Foundation found 63% of patients were happy with their biosimilar. But 28% were upset they weren’t told about the switch. Transparency matters. Many patients feel blindsided when their medication changes without warning. Even if the drug is safe, the psychological impact of an unexpected switch can affect how well people stick with treatment. Some doctors worry about this. Dr. Kevin Winthrop from Oregon Health & Science University found that psoriasis patients switched to biosimilars without consent were 20% more likely to stop treatment entirely. That’s not because the drugs don’t work. It’s because trust was broken.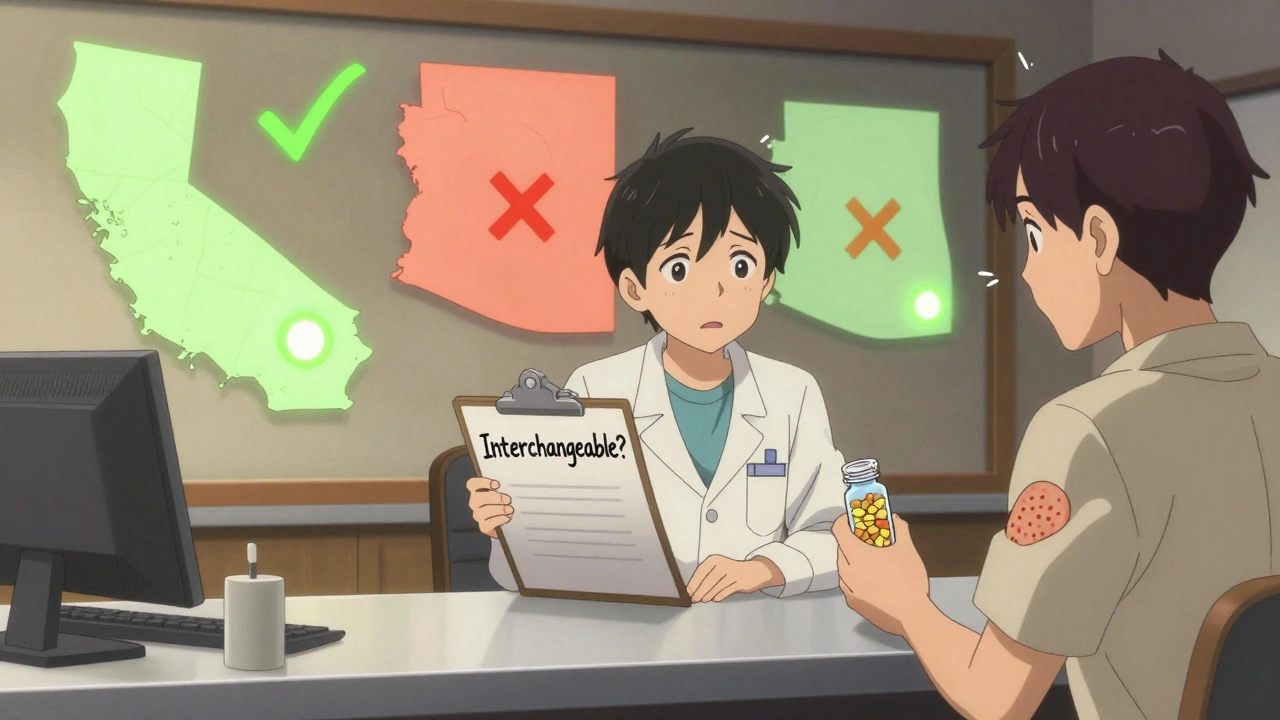
Why Does This Even Matter?
Biologics are expensive. Humira, for example, costs over $7,000 a month without insurance. Biosimilars are priced 15% to 30% lower. Interchangeable ones sell faster. Semglee hit 17% market share in six months. Non-interchangeable biosimilars took much longer to catch on. The goal is simple: lower costs and more access. In states with automatic substitution, insulin biosimilar use jumped 18.7% compared to states without those laws. That’s real savings-for patients, insurers, and the health system. But there’s a trade-off. If patients stop taking their meds because they’re confused or anxious about the switch, the cost savings vanish. And if pharmacies can’t keep up with the rules, mistakes happen.What’s Next?
A bill called the Biosimilar Red Tape Elimination Act was introduced in 2022. It wants to scrap the switching studies and make all FDA-approved biosimilars interchangeable. The biotech industry (PhRMA) says that’s dangerous. The biosimilar industry says it’s long overdue. The FDA is also looking at streamlining the switching study requirements. They might accept data from fewer patients or shorter studies. That could speed up how many biosimilars become interchangeable. By 2025, 70% of the top-selling biologics will have biosimilar competitors. Interchangeability will become a make-or-break factor for market success. Pharmacies, insurers, and patients will need clearer rules-and better communication.What Should You Do?
If you’re on a biologic, ask your doctor: "Is there a biosimilar option? Is it interchangeable?" Don’t assume your pharmacist will tell you. Ask your pharmacy: "Will I be notified if my medication changes?" Keep a list of the exact name and manufacturer of your drug. If you notice new side effects after a switch, tell your doctor immediately. Pharmacists: Know your state’s laws. Use the American Pharmacists Association’s free training. Document every substitution. Talk to patients. Don’t assume they understand what’s happening. The system is designed to save money and improve access. But it only works if everyone-patients, doctors, pharmacists-understands how it works. Clarity beats convenience. Communication beats confusion.Can any biosimilar be swapped for the original drug without a doctor’s permission?
No. Only biosimilars that have received FDA interchangeability designation can be automatically substituted. Not all biosimilars have this status. Even if a biosimilar is approved, it may still require a doctor’s approval depending on your state’s laws.
Are interchangeable biosimilars safer or more effective than regular biosimilars?
No. All FDA-approved biosimilars, whether interchangeable or not, meet the same high standards for safety and effectiveness. The interchangeability label only means the drug has passed extra tests showing it’s safe to switch back and forth with the original biologic. It doesn’t mean it’s better.
Can a pharmacist switch me from one biosimilar to another without telling me?
No. FDA rules don’t allow automatic substitution between two biosimilars-even if both are interchangeable. Switching from one biosimilar to another requires a new prescription from your doctor. This is a common source of confusion in pharmacies.
Why do some states block automatic substitution?
Some states require prescriber approval to protect patient safety, especially for complex conditions like rheumatoid arthritis or psoriasis. Others want to ensure substitution only happens if it lowers costs. These rules reflect ongoing debates about patient autonomy, cost control, and clinical risk.
What should I do if I’m switched to a biosimilar without my knowledge?
Check your medication label for the manufacturer name and product name. If it’s different from what your doctor prescribed, contact your pharmacist and doctor. Report any new side effects. You have the right to ask for your original medication back. Many states require pharmacists to notify you before substitution-so if you weren’t told, that’s a violation.

Comments (10)
John Webber
December 2, 2025 AT 08:18
so like... if my insulin gets swapped without me knowing and i start feeling weird, its my fault for not checking the label? smh. they say its safe but then people get allergic to the fillers?? why dont they just tell us??
Shubham Pandey
December 3, 2025 AT 13:33
too much text. just tell me if i can swap or not.
Elizabeth Farrell
December 4, 2025 AT 08:40
I really appreciate how thorough this breakdown is. It’s easy to feel overwhelmed when your medication changes without warning, but knowing the difference between biosimilars and interchangeable ones helps me have better conversations with my pharmacist. I always ask for the manufacturer name now - it’s a small step, but it makes me feel more in control. And if you’re scared about switching? That’s valid. Your feelings matter as much as the science.
Sheryl Lynn
December 4, 2025 AT 11:34
Oh, darling, the FDA’s ‘interchangeable’ label is less a scientific triumph and more a corporate loophole dressed in regulatory lace. We’re not talking about aspirin here - we’re dealing with living, breathing, cellular alchemy that even the most sophisticated labs can’t perfectly replicate. To call these ‘equivalent’ is to confuse statistical noise with biological truth. And don’t get me started on state laws - it’s like a federalist fever dream where pharmacy techs are expected to be constitutional scholars.
Paul Santos
December 5, 2025 AT 10:27
Interchangeability is essentially a cost-driven epistemological compromise 🤷♂️. We’ve reduced complex biologics to a binary: ‘safe enough to swap’ vs ‘not quite’. But biology doesn’t do binaries - it does gradients, noise, and emergent properties. The FDA’s criteria are pragmatic, sure, but they’re also a form of epistemic violence against the patient’s lived experience. Also: 🤖💊
Eddy Kimani
December 5, 2025 AT 20:04
Interesting how the switching studies focus on pharmacokinetics but ignore immunogenicity over time. Most patients aren’t just switching once - they’re cycling through multiple biosimilars over years. Are we really sure the cumulative effect is neutral? The data seems thin. Also, why isn’t there more research on how patient trust impacts adherence? That’s a huge confounder.
Chelsea Moore
December 6, 2025 AT 11:06
HOW DARE THEY?!?! They just swap my Humira for some cheap knockoff without telling me?!?! I could’ve died!! My psoriasis flared up for THREE WEEKS!! And now they want to make ALL biosimilars interchangeable?!?! This is a medical horror story!! Someone needs to sue!!
John Biesecker
December 6, 2025 AT 19:30
you know, i used to think medicine was just chemicals and science... but this? this is about trust. it’s about how we treat people who are already scared. if i’m paying $7k a month for a drug, i deserve to know if it’s changed - not because it’s less safe, but because it’s *mine*. and maybe that’s the real problem: we’re treating patients like numbers, not humans 🌱
Genesis Rubi
December 6, 2025 AT 21:25
Why are we letting other countries dictate our healthcare? We invented biologics. We have the best scientists. Why are we letting some Indian lab make a copy and call it interchangeable? This is weak. We need to protect American innovation, not hand it out like free samples. And don’t even get me started on state laws - this is America, not a patchwork quilt of confusion.
Saravanan Sathyanandha
December 8, 2025 AT 05:03
As someone from India where biosimilars have been used for over a decade, I can say this: transparency is the real medicine here. In our system, pharmacists are trained to explain the switch - not just legally, but compassionately. The science is solid. The fear? That’s cultural. Maybe instead of changing laws, we need to change how we talk to patients. A simple conversation beats a thousand regulatory forms.